MDR Third Amendment is official: EUDAMED Mandatory Use by January 2026
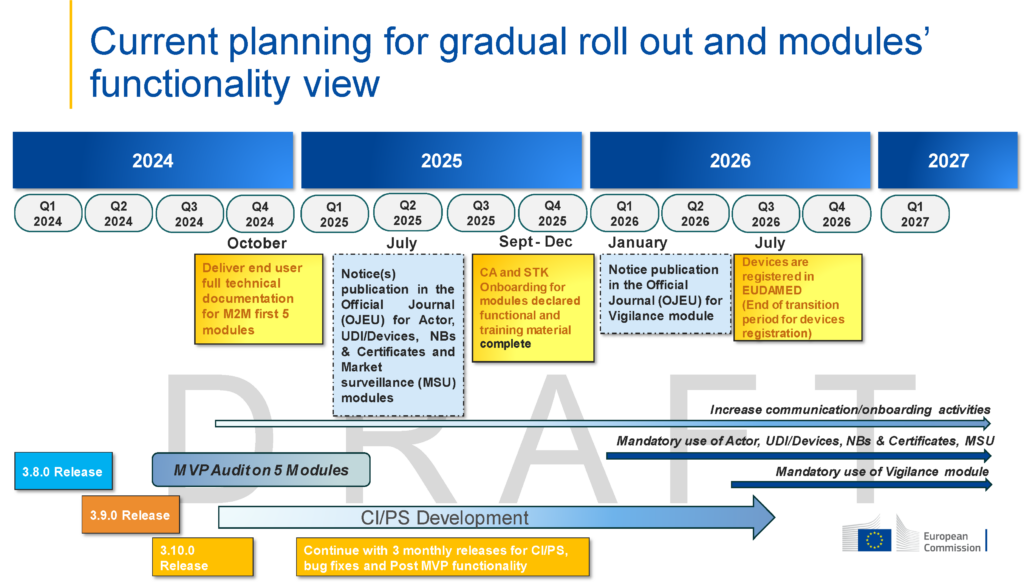
Source: EU Commission | Directorate-General for Health and Food Safety | Sante > Updated Timeline – Current planning for gradual roll out and modules’ functionality view
With the Third Amendment to the European Union’s Medical Device Regulations (MDR) now officially ratified by the EU Parliament, the timelines for EUDAMED roll-out have substantially changed. Further, the EU Commission is now taking a modular approach to rolling out its EUDAMED system, starting with three modules being available for mandatory use by January 2026.
The EU Commission has allowed for a six (6) month transition period so that all devices must be submitted for registration by July 2026. The first three modules to go-live for mandatory use are:
- Actor Registration for Economic Operators (medical device manufacturers, authorized representatives, importers etc.)
- Notified Bodies & Certificates for Notified Bodies (NBs) to uploading conformity assessment data and certificates for approved devices.
- Device Registration (UDI) for device manufacturers and/or their authorized representatives to submit device data for registration.
The Vigilance and Post Market Surveillance module (V/PMS) is expected to be ready for mandatory use by Q3 2026.
How Innovit Can Help You
Innovit is a reliable and valuable ally for companies navigating the complexities of EUDAMED data compliance and global device registration tracking. We continuously stay on top of the changing UDI landscape through our partnerships with medical device companies and MedTech industry associations. With our extensive experience in Healthcare and Life Science, focusing on master data management and UDI compliance, we bring insights that are not yet in the public arena, helping our customers stay ahead of the UDI regulatory curve.
