MDR and Class I Medical Devices – What’s In, What’s Out?
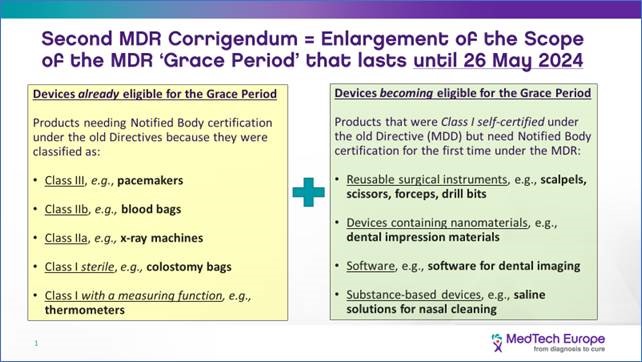
European Commission announced last October that the new EUDAMED will not launch until May 2022, giving everyone enough time to properly prepare for implementing M2M data exchange with EUDAMED. Further, a grace period was also introduced by the EU for certain classes of devices. See the diagram below from MedTech Europe.
Three different sub-classes of Class I medical devices will get an additional four years to come into compliance with the new regulation. Devices in full compliance with the current Medical Device Directive (MDD) that are either (1) reusable surgical instruments, (2) in sterile conditions, or (3) have a measuring function, may remain or be placed on the market until 26 May 2024. Quite simply, there’s more time (May 2024 instead of May 2020) to get a first time Notified Body approval for devices which will be up-classified into risk-classes Is (sterile), Im (measuring function) or Ir (reusable surgical instruments).
Why Extend the Deadline?
Previous regulations excluded many Class I devices. However, an enormous number of devices were ‘up-classified’ requiring a conformity assessment under the MDR date of 26 May 2020. Several major challenges prevented manufacturers and their devices from being (re-)certified against the new rules of the Medical Device Regulation (MDR) such as severe lack of Notified Body capacity, expert panels and EU guidance. Certification by May 2020 for a significant number of medical devices would have been difficult to achieve, if not impossible. On the other hand, forcing the removal of those devices from the market would have been unacceptable.
What Happened?
The 2nd Corrigendum extended the deadline until May 2024 for ‘up-classified’ Class I devices (measuring, re-usable surgical, sterile and software) and created a new risk class for the remaining Class I devices still required to meet the May 2020 deadline. These devices include non-sterile, non-measuring devices that are self-assessed under MDD guidelines.
Bottom line: manufacturers of reusable surgical instruments, devices that have a measuring function and devices with software, will have an additional four years to meet the stricter requirements of MDR.
Which Class I devices MUST STILL meet May 2020 deadline?
Class I non-measuring or non-sterile medical devices – which account for about 10% of Class I devices – are still obligated to meet the 26 May 2020 deadline.
The diagram below from MedTech Europe is a good summary of current MDR application dates.
Next Steps
Quite frankly, medical device manufacturers have two choices. Do nothing and hope to slip through unnoticed, risking non-compliance and lost revenue. Or commit to a solid tactical plan for solution development and prepare your data for submission to EUDAMED. We recommend starting early to take advantage of this addition grace period and prepare properly – ensuring high standards of data quality and minimizing the costs associated with rushed implementations.
