AusUDID OVERVIEW
What is AusUDID?
The Australian Unique Device Identification Database (AusUDID) provides free public access to information on medical devices supplied in Australia. The AusUDID:
- Stores Unique Device Identifiers (UDI-DIs) and related information for each medical device.
- Links each device to its official entry in the Australian Register of Therapeutic Goods (ARTG).
- Requires sponsors and manufacturers to submit and maintain their device data.
- Allows patients, consumers, health professionals, and clinical registries to freely access device details.
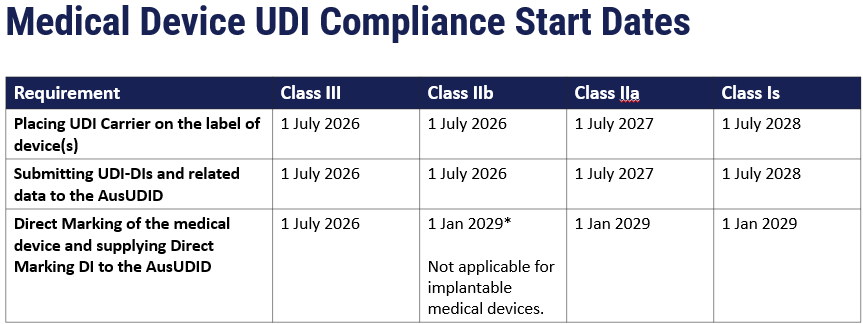
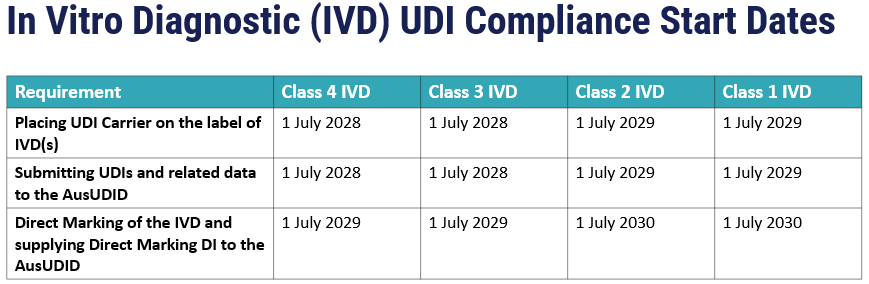
Purpose of AusUDID
Enhance patient safety by enabling rapid identification and response to adverse events, safety alerts, or recalls involving medical devices.
Improve access to device information for patients, healthcare professionals, and consumers.
Enable better device performance evaluation through accurate product tracking, regulatory analysis & clinical studies.
Align Australia with global standards for medical device identification, promoting consistency and interoperability across international health systems.
Did you know?
Mandatory device submission to AusUDID starts in July 2026 for high-risk and implanted medical devices, with other devices phased in gradually based on their risk class.
