GUDID OVERVIEW
What is GUDID?
The Global Unique Device Identification Database (GUDID) is a database administered by the FDA with the purpose of centralizing and making accessible key medical device information for every device with a unique device identifier (UDI) on its label.
UDI is made up of a device identifier (DI) that identifies the labeler and a specific model of a device and a production identifier (PI) that is variable depending on the specific production of the device. A production (PI) can be a lot/batch number, serial number, expiration date, manufacturing date or distinct identification code (for a human cell, tissue, or cellular and tissue-based products that are regulated as a device).
GUDID contains ONLY the device identifier (DI) portion of the UDI, which serves as the primary key to obtain medical device information in the database. With this information, the GUDID aims to ensure patient safety, facilitate post-market surveillance and streamline the safe distribution of medical devices in the US market.
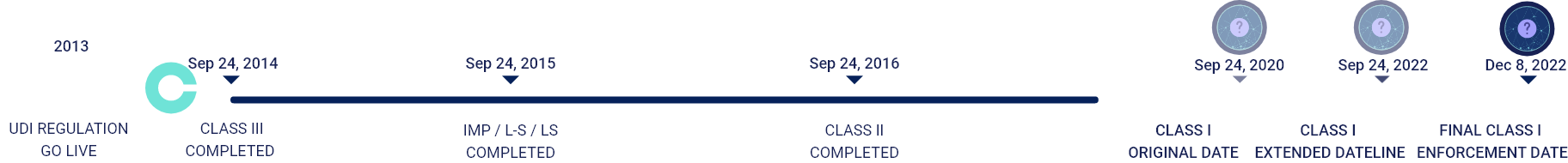
GUDID Data Submission Timeline
SEPTEMBER 24, 2014
Medical Devices & Devices Licensed under the Public Health Service Act
SEPTEMBER 24, 2016
CLASS III
Implantable, Life-Supporting & Life Sustaining Medical Devices
Implantable, Life-Supporting & Life Sustaining Medical Devices
SEPTEMBER 24, 2018
CLASS II
Implantable & Medical Devices
Implantable & Medical Devices
SEPTEMBER 24, 2022
CLASS I
Low risk & Unclassified Medical Devices
Original class I submission deadline was September 2020 but due to COVID19 pandemic the FDA postponed its deadline to September 2022
Purpose of GUDID
Improve patient safety and reduce medical errors through rapid and accurate identification of medical devices
Enhance overall transparency of medical device information to the public and healthcare providers about devices placed in the US market
Enable more accurate reporting and analysis on medical device data
Establish more robust post-market surveillance and recall activities
Facilitate efficient and reliable collaboration between manufacturers, distributors, GPOs, and hospitals through a standardized identification of medical devices
Did you know?
FDA will enforce UDI compliance dates for Class I and unclassified devices beginning Sept 24, 2022 (two years after the previous target of Sept 24, 2020)
