
by Innovit Product Team | Apr 19, 2020 | Blog, GUDID, UDI
The Global Unique Device Identification Database (GUDID) is a database administered by the FDA that will serve as a reference catalog for every medical device with an identifier. Unique Device Identification (UDI) is a system to mark and identify medical devices (via...
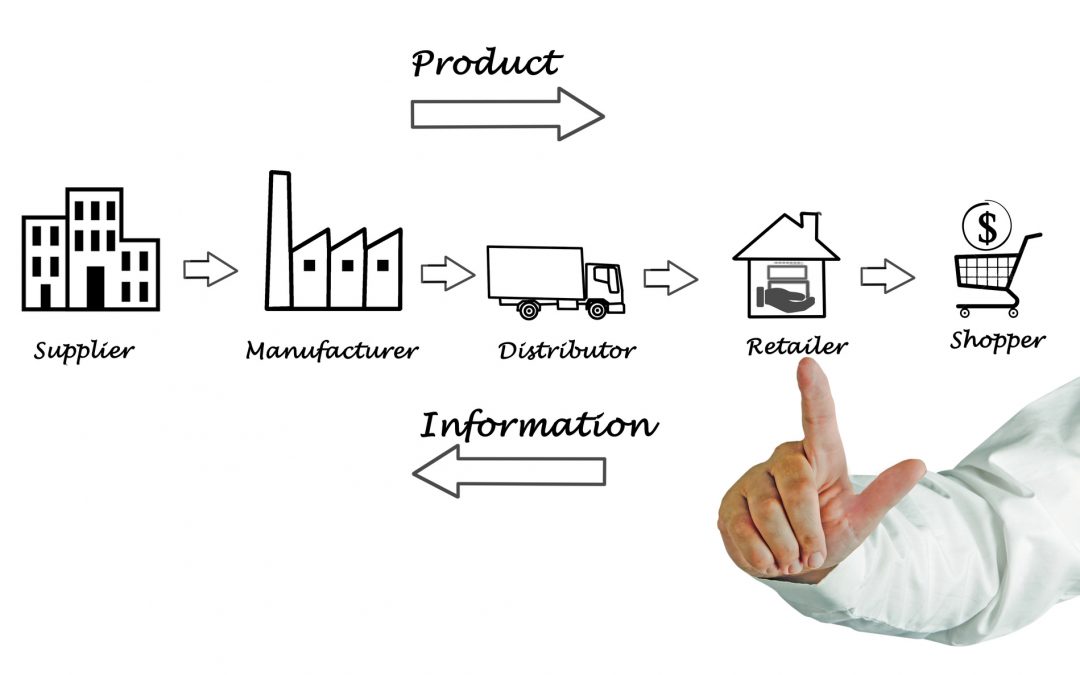
by Innovit Product Team | Apr 25, 2019 | Blog, GDSN
Ecommerce sales will keep rising; brick-and-mortar retail sales will keep declining. An important factor to consider here is the generational shift. As the “digital native” Generation Z enters the workforce, their spending power will increase, and it’s safe to assume...

by Innovit Product Team | Apr 2, 2019 | Blog, GS1
All eyes are on the U.K. this week as they continue navigating missed deadlines surrounding Brexit. The news isn’t reassuring as reports indicate that business sentiment is on the decline as Brexit uncertainty continues. Yesterday, it was announced that the...

by Innovit Product Team | Mar 12, 2019 | Blog, UDI
Manufacturers of medical devices and companies that supply them must provide products and services that are both safe and effective. And today’s mounting list of regulations, medical device compliance is important to the success of every medical device manufacturer....

by Innovit Product Team | Mar 5, 2019 | Blog, NPI
Developing and following a robust NPI process and workflow can make the difference between success and failure. An NPI workflow helps boost sales, customer satisfaction and revenue growth. The ideal NPI process is comprised of a set of systematic steps that help...






