by Innovit Corporate Affairs | Sep 24, 2019 | Blog, PIM
There’s a mounting list of regulations aimed towards Unique Device Identification (UDI), intended to improve patient safety, supply chain efficiency and post-market surveillance. The benefits are numerous, but challenges continue for medical device suppliers on their...

by Innovit Corporate Affairs | Aug 20, 2019 | Blog
We pride ourselves on being experts in MDM and PIM. But we also pride ourselves in keeping up with new technologies and industry trends. Today, we’re taking a long view – after all, why look to 2020 when we can leapfrog to 2040 to reveal a potentially...
by Innovit Corporate Affairs | Jun 17, 2019 | Blog, GDSN
Join Innovit June 19-21 in Denver, CO The GS1 Connect 2019 conference in Denver, CO kicks off THIS WEEK and we’re ready for three days of thought-provoking sessions, collaboration and networking opportunities. It’s a great event – and a perfect opportunity for...
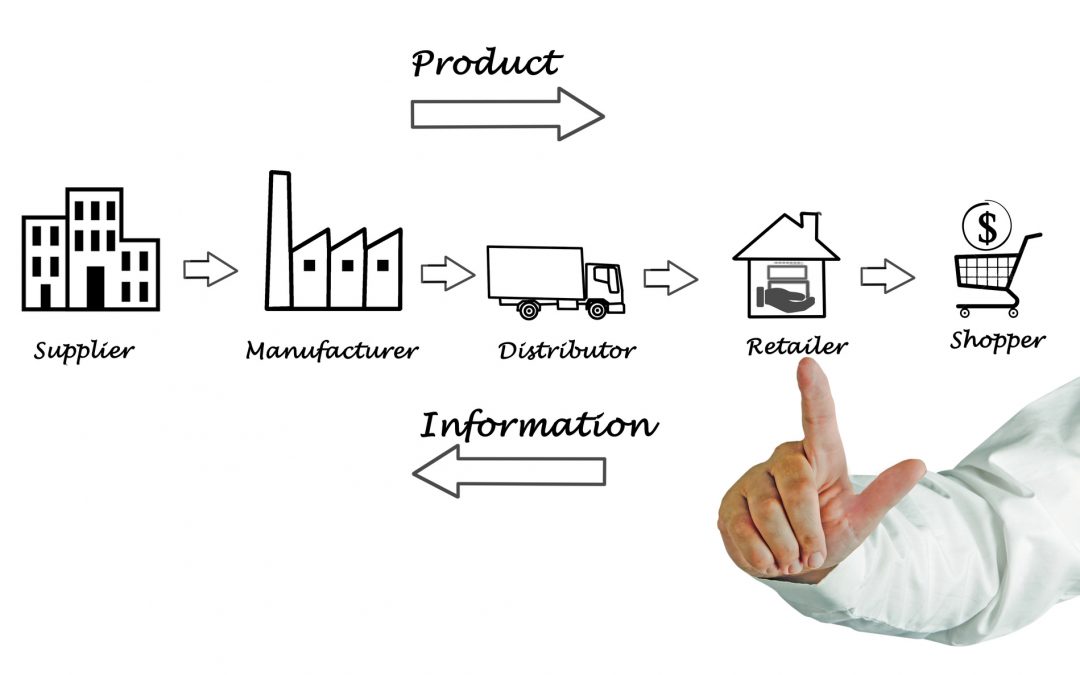
by Innovit Product Team | Apr 25, 2019 | Blog, GDSN
Ecommerce sales will keep rising; brick-and-mortar retail sales will keep declining. An important factor to consider here is the generational shift. As the “digital native” Generation Z enters the workforce, their spending power will increase, and it’s safe to assume...
by Innovit Corporate Affairs | Apr 15, 2019 | Blog
Innovit, a global MDM and PIM solutions, has been accepted into Medtech Europe and the Medtech Europe Corporate Council as a corporate associate member. The Council is an invitation-only community representing In-Vitro Diagnostics and Medical Devices manufacturers...


