Mistake by Florida on child COVID-19 rate raises the question: Can Florida's numbers be trusted? “It’s unacceptable to publish information that changes so dramatically that it warrants explanation, and then to not provide any...
Blog
Get the latest Innovit content today to help you with your business transformation.
FDA Delays UDI Deadline for Class I Devices to September 2022
The US Food and Drug Administration (FDA) has released new guidance regarding Unique Device Identification (UDI) compliance dates for Class I and unclassified medical devices. Effective immediately, the FDA has postponed GUDID (Global UDI...
EU-MDR Date of Application – Postponed…
The European Parliament has formally voted and adopted the amendment of its Medical Device Regulation (MDR) date of application. The Parliament has tabled and supported the four technical amendments proposed...
GUDID and UDI – Are You Prepared?
The Global Unique Device Identification Database (GUDID) is a database administered by the FDA that will serve as a reference catalog for every medical device with an identifier. Unique Device Identification (UDI) is a system to mark and identify...
Healthcare Providers and COVID-19: Flattening the Curve
On March 11, 2020, the World Health Organization designated “coronavirus disease 2019” (COVID-19) a global pandemic. As the number of cases worldwide continues to grow exponentially, social distancing has become the norm to slow the rate of...
Medical Device Manufacturing and COVID-19: Challenges & Opportunities
Medical Device manufacturers are grappling with disruptions to their businesses as a result of the coronavirus pandemic. Many are experiencing adverse impact to their supply chain and operations, financial expectations and emergency response...
URGENT: MDR Implementation – Postponed by EU Commission
The European Commission has announced a proposal to delay enforcement of the EU Medical Device Regulation by one year, from 26 May 2020 to 26 May 2021. The intent is to help relieve pressure from national competent authorities, notified bodies,...
EUDAMED: Regulation News & Updates
The European Commission recently announced last November that the new EUDAMED will not launch until May 2022, even as the MDR takes effect in May 2020. While the delay in EUDAMED certainly give us more time before we must submit information to the...
MDR and Class I Medical Devices – What’s In, What’s Out?
European Commission announced last October that the new EUDAMED will not launch until May 2022, giving everyone enough time to properly prepare for implementing M2M data exchange with EUDAMED. Further, a grace period was also introduced by the EU...
Is GHX Discontinuing Support for GUDID and EUDAMED?
In September 2019, we witnessed PLM software giant, PTC Windchill, quietly exit the UDI compliance market. As we ended 2019, several little birds have shared that GHX is slated to phase out support for their UDI Data Submission Service and will...
Healthcare Insights – 2019 Recap
Happy New Year! As the dust settles from the holiday madness and end-of-year celebrations, we thought we’d take a quick look back and reflect upon our 2019 journey. We’re so fortunate to work with an amazing roster of customers and partners whose...
EUDAMED – Deadlines Updated
The European Commission recently announced that the new EUDAMED will not launch until May 2022, even as the MDR takes effect in May 2020. Given this postponement, we now have the opportunity, and sufficient time, to properly prepare for...
The Demerger of 1WorldSync & Atrify: How it Impacts FDA GUDID Users
The beginning of July brought several major announcements in the product content and data synchronization market. The joint venture between GS1 Germany and GS1 US effectively ended with the demerger from 1WorldSync, which has evolved as follows:...
EUDAMED Go-Live is March 2020: Why waiting for more Technical Specifications is NOT a winning strategy
EUDAMED deadlines remain unchanged – the go-live date is still March 26, 2020 with the Date of Application for device registration/submission standing at May 26, 2020. Yet, we’re nearing the end of 2019 with most med device companies still...
Healthcare Insights: PIM vs PLM – What’s the Best Method to Address Regulatory Data Submission Challenges?
There’s a mounting list of regulations aimed towards Unique Device Identification (UDI), intended to improve patient safety, supply chain efficiency and post-market surveillance. The benefits are numerous, but challenges continue for medical device...
Is PTC Windchill Exiting the UDI Compliance Market?
If you’re a medical device manufacturer or supplier submitting data to the FDA’s GUDID system, then your life may be getting a bit more complicated if you’re also using PTC’s UDI solution to comply with this FDA regulation. Recent announcements...
Success Spotlight: GPC Asia Pacific (Repco)
Today’s Success Spotlight With over 13,000,000 vehicle parts relationships, GPC Asia Pacific (Repco), a leading provider of automotive aftermarket parts and accessories, manages a catalog of over 1,300,000 items with approximately 50,000 vehicle...
Healthcare Innovation in 2040: The Next Big Thing in Patient Care
We pride ourselves on being experts in MDM and PIM. But we also pride ourselves in keeping up with new technologies and industry trends. Today, we're taking a long view - after all, why look to 2020 when we can leapfrog to 2040 to reveal a...
Success Spotlight: Global Medical Device Supplier
The goal of UDI is to help improve patient safety by enabling better traceability of medical devices consumed. For the ‘labeler’, UDI will help improve internal processes, bolster master data quality, and provide more accurate reporting and...
What is the best path for UDI compliance? Examining Two Approaches for Medical Device Suppliers
As a global software solution vendor in Life Sciences & Healthcare, our customers include many of the top medical device suppliers managing high volumes of product data and tens of thousands of device records. Based upon our experience helping...
Healthcare Insights: Medical Device Regulation Updates – What You Need to Know
Keeping up with the evolving medical device regulation and compliance landscape can be challenging, particularly when technical specifications from the EU Commission are repeatedly delayed. As a global software solution vendor for Life Sciences...
Eudamed: Technical Specifications Update
Keeping up with the evolving medical device regulation and compliance landscape can be challenging, particularly when technical specifications from the EU Commission are repeatedly delayed. As members of MedTech Europe and Healthcare...
Key Takeaways from GS1 Connect 2019 – Dynamic Innovation & Outstanding Leadership
Last month, over 1,200 guests traveled to Denver, Colorado for GS1 Connect 2019, the annual 3‑day conference bringing IT and supply chain partners together to learn best practices for leveraging GS1 Standards that drive innovation and better...
Point of Care Scanning at Hospitals – Why Trusted Data is Key
Healthcare providers are increasingly putting emphasis on a patient-centric approach to care delivery. From ensuring patient safety to providing quality care at an affordable cost, care providers have embraced healthcare information technologies...
We’re Here – GS1 Connect 2019!
Join Innovit June 19-21 in Denver, CO The GS1 Connect 2019 conference in Denver, CO kicks off THIS WEEK and we're ready for three days of thought-provoking sessions, collaboration and networking opportunities. It’s a great event – and a perfect...
FDA’s GUDID Medical Device Data Corrections – Implications of the Shortened Grace Period
In 2014, the seven-day grace period for Global Unique Device Identification Database (GUDID) submission corrections was extended to 30 days. Over time, healthcare providers provided feedback stating that 30 days is too long to wait for access to...
Key Takeaways from the Europe Commission’s New EUDAMED Data Exchange Guidelines
As it prepares to meet new regulatory requirements in Europe, the medical device community is following developments regarding the European Database for Medical Devices (Eudamed). Most recently, the European Commission (EC) released three...
The Latest UDI News: Convenience Kits Q&A from the FDA
Recently, the FDA issued the final guidance, “Unique Device Identification: Convenience Kits” to help clarify the FDA’s policy related to UDI considerations for medical procedure kits. The intent of this guidance is to ensure that the exception for...
Why eCommerce Success Demands GDSN Connectors
Ecommerce sales will keep rising; brick-and-mortar retail sales will keep declining. An important factor to consider here is the generational shift. As the “digital native” Generation Z enters the workforce, their spending power will increase, and...
Exciting News – Innovit Accepted into Medtech Europe!
Innovit, a global MDM and PIM solutions, has been accepted into Medtech Europe and the Medtech Europe Corporate Council as a corporate associate member. The Council is an invitation-only community representing In-Vitro Diagnostics and Medical...
Talking Brexit at the GS1 UK Healthcare Conference, London UK, 09-10 April 2019
All eyes are on the U.K. this week as they continue navigating missed deadlines surrounding Brexit. The news isn’t reassuring as reports indicate that business sentiment is on the decline as Brexit uncertainty continues. Yesterday, it was...
We’re Here! Global GS1 Healthcare 2019
Join Innovit on 26 - 29 March in Noordwijk-Amsterdam, The Netherlands The Global GS1 Healthcare Conference, 26-28 March 2019, in Noordwijk-Amsterdam, the Netherlands kicks off TODAY and we're very excited to get up-to-date on the newest global...
Complying with UDI & MDR: Facts, Figures and Action Items
Manufacturers of medical devices and companies that supply them must provide products and services that are both safe and effective. And today’s mounting list of regulations, medical device compliance is important to the success of every medical...
New Product Introduction – How an NPI Workflow is the Key to a Winning Process
Developing and following a robust NPI process and workflow can make the difference between success and failure. An NPI workflow helps boost sales, customer satisfaction and revenue growth. The ideal NPI process is comprised of a set of systematic...
EUDAMED & MDR: Readiness Tips
With a May 2021 go live date, The European Database for Medical Devices (EUDAMED) will be open to many more groups, including manufacturers, importers and sponsors. Current estimates are that this covers 70,000+ individual organizations and over...
No-Deal Brexit – Implications to Medical Device Regulations (MDR)
A no-deal Brexit (a disaster to some or the chance trade on our own terms to others) means the UK would leave the European Union (EU) immediately on 29 March 2019, and there would be no agreements in place about what their relationship would be...
Latest developments on GDSN and Master Data Excellence. Innovit heads to Brussels for the GS1 Global Forum.
The GS1 Global Forum, scheduled to kick-off next week on February 18, 2019 in Brussels - is GS1’s largest global annual event aimed at aligning the organization’s strategy while strengthening the GS1 staff around the world. The week offers great...
Leveraging Your Wealth of Healthcare Data for Better Patient Care
Innovit at GS1 UK Healthcare Conference, London Managing the wealth of available healthcare data allows health systems to create holistic views of patients, personalize treatments, improve communication and enhance health outcomes....
Innovit, GS1 Global Healthcare Conference and new EUDAMED & MDR Challenges
We're attending the GS1 Global Healthcare Conference! Master data is at the center of important initiatives within healthcare including patient safety, post-market surveillance, supply chain optimization and regulatory compliance. While...
5 Real-World Ways Brands Leverage Customer Data Analysis to Grow
Data-driven organizations are 23 times more likely to acquire customers, 6 times as likely to retain customers, and 19 times as likely to be profitable as a result. That's because there's nothing more powerful than knowing how to leverage customer...
What Is PIM and How Does It Improve the Food and Beverage Industry?
The food and beverage industry is divided into two major segments. One of those is production and the other is the distribution of edible goods. Marketing software plays a big role in digital marketing strategy as well as the management for online...
7 Habits of Highly Effective Master Data Management Solutions
Your company's master data must be protected. If it isn't, your business may suffer greatly, or completely fail to operate. As part of that oh-so-important protection, adequate and effective management needs to be in place. So how is your master...
How to Use CPG Data to Win Customers and Influence Sales
If you want to get more people to choose your brand over another brand, there are ways to make data-driven decisions that gain customers. Paying attention to old data isn't going to provide the results you want. You need real-time data to get the...
Understanding The Value of MDM Tools and The Impact on Your Business
If you've thought the amount of data your organization is dealing with has been increasing, it's because it is. The numbers prove it. Over 2.5 quintillion bytes of data are created every day, and the number is growing. Experts predict every person...
The Future of GDSN – How It Will Impact Your Business
According to Veritas, only 15% of data out there can be classified as Business Critical Information. This excess data could cost $3.3 trillion in storage and management by the year 2020. The reason why so much of this data is unaccounted for is...
Healthcare IT Solutions: The Role of PIM in Healthcare
Product information management (PIM) systems are expected to grow up to 25.5% by 2023. There is even a growing demand for PIM in the medical field. Specifically, this interest lies in healthcare IT solutions. But what's the hype all about? Why are...
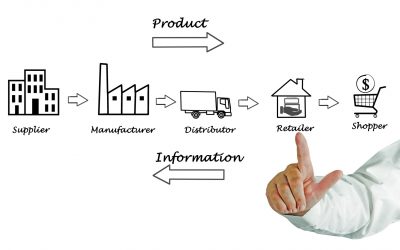
Healthcare Supply Chain Management: How It Impacts The Patient Experience
According to a recent survey, over 69% of Healthcare leaders in IT emphasized on healthcare supply chain management as they believe it is an important factor for actionable data mining. In addition to that, with over $672.1 billion spent on...
What is PIM Software and How Can It Help You?
Once upon a time, businesses had to keep paper files detailing everything about their products and customers. But when the internet came into the picture, that all changed. Now, businesses are finding ways to manage vast amounts of data...
New Product Introduction Technology: How it Can Help Your Business
Every year, over 30,000 new consumer products are launched and 80% of them fail. Launching a new product successfully involves a lot of interrelated tasks that all require a high degree of attention. This involves testing, cost-effective production...
How PIM and MDM Can Help Regional Grocery Stores Succeed
Half of Americans are avoiding the grocery store entirely and purchasing their goods online. That percentage is expected to increase in 2018. With more consumers turning to the internet and delivery services for their groceries, it's no wonder why...